Wholesale Tirzepatide Powder from Leading Manufacturers
As a trusted supplier in the industry, I understand the growing demand for high-quality Tirzepatide powder. This innovative compound is making waves in the market, and I offer it at competitive wholesale prices. Sourced directly from reputable manufacturers, our Tirzepatide powder meets rigorous quality standards, ensuring you receive a reliable product for your business needs. Whether you’re formulating for diabetes management or weight loss solutions, our Tirzepatide powder serves as an excellent ingredient, enhancing your offerings. I pride myself on providing exceptional customer service, helping you navigate pricing and logistics for bulk orders. With me, you can rest assured you’re partnering with someone who values your success as much as I do. Let’s work together to elevate your product line and satisfy your clients with effective solutions using Tirzepatide powder. Reach out today to discuss how we can meet your wholesale needs and take your business to the next level!
Tirzepatide powder Stands Out Industry Giant
Tirzepatide powder is making waves in the pharmaceutical industry, particularly in the realm of diabetes treatment and weight management. As a dual-action glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, Tirzepatide not only enhances insulin secretion but also plays a vital role in regulating appetite. This innovative mechanism positions it as a standout choice among current therapeutic options, making it attractive to global procurement buyers seeking effective pharmaceutical ingredients. The demand for Tirzepatide powder has surged due to its impressive efficacy and safety profile, prompting manufacturers to explore partnerships with suppliers who can provide high-quality compounds. Quality assurance remains paramount, as procurement professionals need to ensure consistency, purity, and reliable sourcing of Tirzepatide. This presents an excellent opportunity for suppliers to differentiate themselves by adopting stringent quality control measures and demonstrating compliance with international regulatory standards. As the market evolves, the potential for Tirzepatide extends beyond its current applications, with ongoing research and clinical trials exploring its benefits for various metabolic disorders. This opens new avenues for procurement buyers to invest in a product that not only addresses immediate needs but also promises future growth. By aligning with forward-thinking suppliers who prioritize innovation and quality, global buyers can secure a competitive advantage in this dynamic market.
Tirzepatide Powder Stands Out Industry Giant
| Attribute | Description | Value |
|---|---|---|
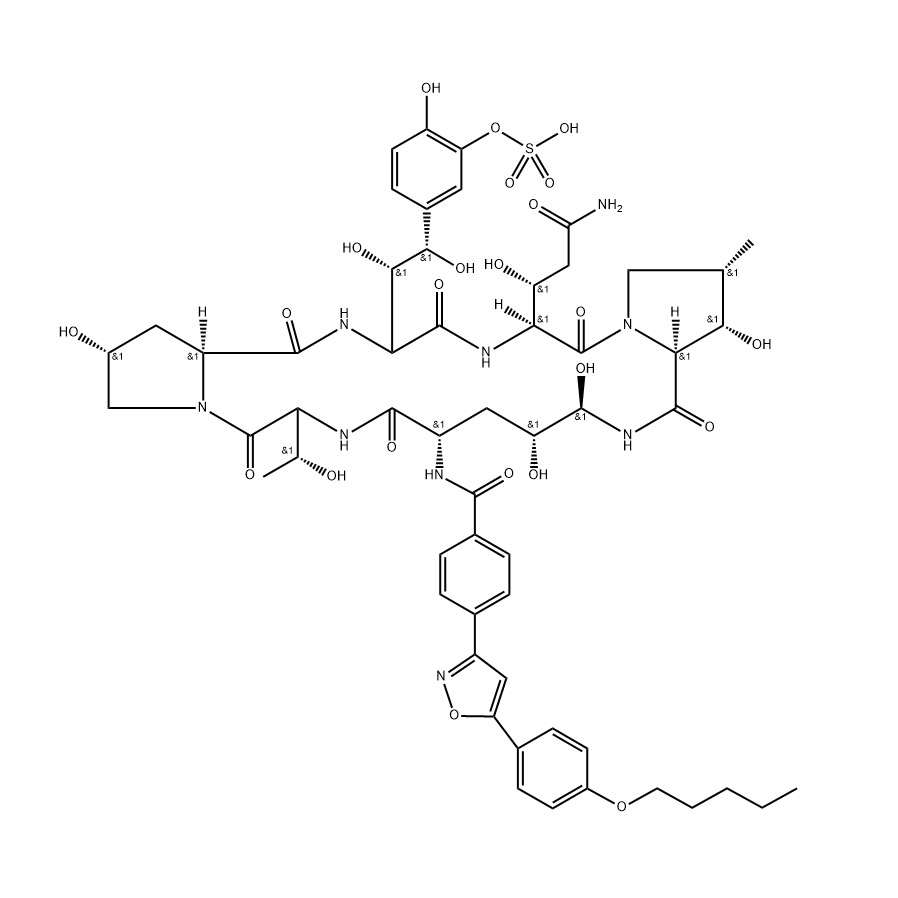
| Molecular Formula | Chemical representation of Tirzepatide | C39H60N10O10S |
| Molecular Weight | Weight of the molecule | 1007.12 g/mol |
| Administration Route | How the drug is administered | Subcutaneous injection |
| Indications | Conditions treated by the medication | Type 2 Diabetes, Obesity |
| Half-Life | Duration drug remains active in the system | 5 days |
| Clinical Trials | Study phases completed | Phase 1, 2, and 3 |
| Market Approval | Regulatory status | FDA Approved |
| Common Side Effects | Adverse reactions reported | Nausea, Vomiting, Diarrhea |
Related Products