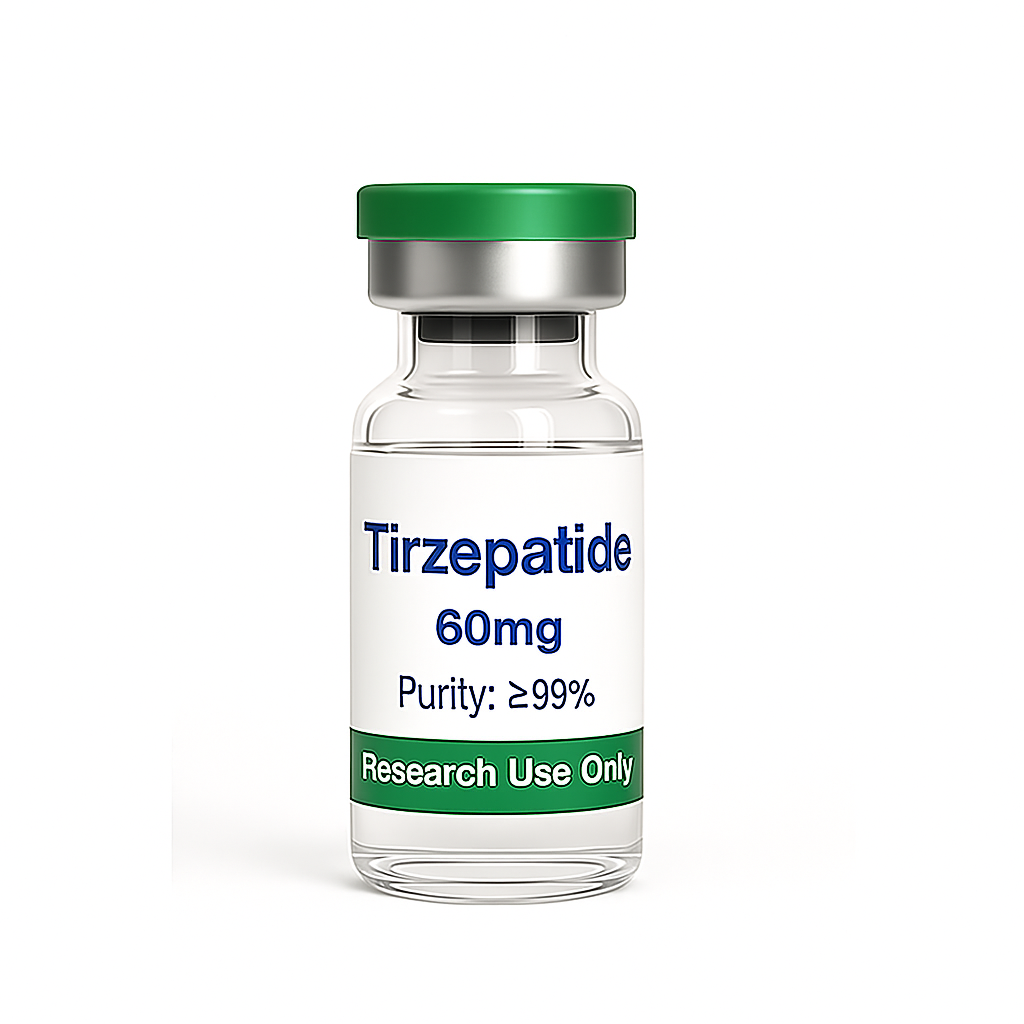
Tirzepatide Lyophilized High Purity 99% Peptide Powder 60mg per vial for diabetes and weight loss
Product Detail
| Name | Tirzepatide Injection Powder |
| Purity | 99% |
| Appearance | White Lyophilized Powder |
| Administration | Subcutaneous Injection |
| Size | 10mg, 15mg, 20mg, 30mg, 60mg |
| Water | 3.0% |
| Benefits | Treating Diabetes, Weight Loss |
Description
Tirzepatide Lyophilized Powder (60 mg)
Tirzepatide (LY3298176) is the first dual-acting agonist that targets both GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1) receptors. It received U.S. FDA approval in May 2022 for the treatment of type 2 diabetes mellitus (T2DM) as an adjunct to diet and exercise.
This product is supplied as a 60mg lyophilized (freeze-dried) sterile powder in vials, which must be reconstituted with bacteriostatic water prior to administration. Compared with single GLP-1 receptor agonists such as semaglutide or dulaglutide, tirzepatide demonstrates superior efficacy in improving blood glucose regulation, enhancing insulin sensitivity, and supporting significant weight loss. These benefits are attributed to its dual-receptor synergistic mechanism of action.
Key Benefits
Glycemic Control
- Stimulates insulin secretion in a glucose-dependent manner
- Reduces glucagon secretion, effectively lowering blood glucose levels
- Mimics the combined metabolic effects of both GIP and GLP-1
Weight Management
- Promotes satiety and reduces caloric intake
- Demonstrated substantial and sustained weight loss in clinical studies
Cardiovascular Health
- Preliminary data indicate potential cardiovascular risk reduction in patients with T2DM
Usage and Dosage
- Tirzepatide lyophilized powder (60 mg) should be reconstituted with bacteriostatic water before use. Administration is by subcutaneous injection, typically once weekly on the same day each week.
Type 2 Diabetes
- Starting Dose: 2.5 mg once weekly
- Titration: Increase every 4 weeks as tolerated
- (→ 5 mg → 7.5 mg → 10 mg → 12.5 mg → 15 mg → 20 mg → 30 mg → 45 mg → up to 60 mg)
- Common Dose: 10–30 mg weekly
- Maximum Dose: 60 mg weekly
Obesity / Weight Management
- Starting Dose: 2.5 mg once weekly
- Titration: Gradual dose escalation as tolerated
- (2.5 → 5 → 7.5 → 10 → 12.5 → 15 → 20 → 30 → 45 → 60 mg)
- Common Dose: 30–60 mg weekly
- Maximum Dose: 60 mg weekly
Recommended Dosage Comparison
| Indication | Starting Dose | Titration Schedule | Common Dose | Maximum Dose | Frequency |
|---|---|---|---|---|---|
| Type 2 Diabetes | 2.5 mg weekly | Increase every 4 weeks (→ 5 → 7.5 → 10 → 12.5 → 15 → 20 → 30 → 45 → 60) | 10–30 mg weekly | 60 mg weekly | Once weekly |
| Obesity / Weight Loss | 2.5 mg weekly | Increase based on tolerance (2.5 → 5 → 7.5 → 10 → 12.5 → 15 → 20 → 30 → 45 → 60) | 30–60 mg weekly | 60 mg weekly | Once weekly |
Note: Ensure each previous dose is well tolerated before escalation.
Possible Adverse Reactions
- Gastrointestinal: Nausea, vomiting, diarrhea, and abdominal discomfort (commonly during initiation)
- Hypoglycemia: Generally low risk, but increased when combined with other antidiabetic medications
- Injection Site Reactions: Redness, itching, swelling, or mild pain at the site
- Pancreatitis: Rare but possible; monitor for persistent abdominal pain
- Renal Effects: Kidney function monitoring may be required in certain individuals
Pharmacokinetics
- Half-Life: Approximately 1 week
- Dosing Frequency: Supports convenient once-weekly administration
Summary
Tirzepatide 60 mg lyophilized powder represents a next-generation therapeutic advancement, combining powerful glycemic control with remarkable weight loss efficacy and potential cardiovascular protection.
With a gradual titration schedule (2.5 mg → up to 60 mg), it allows enhanced tolerability and flexibility for individualized treatment. Its once-weekly administration improves adherence, making it an innovative and effective option for the management of type 2 diabetes and obesity in advanced clinical and research settings.