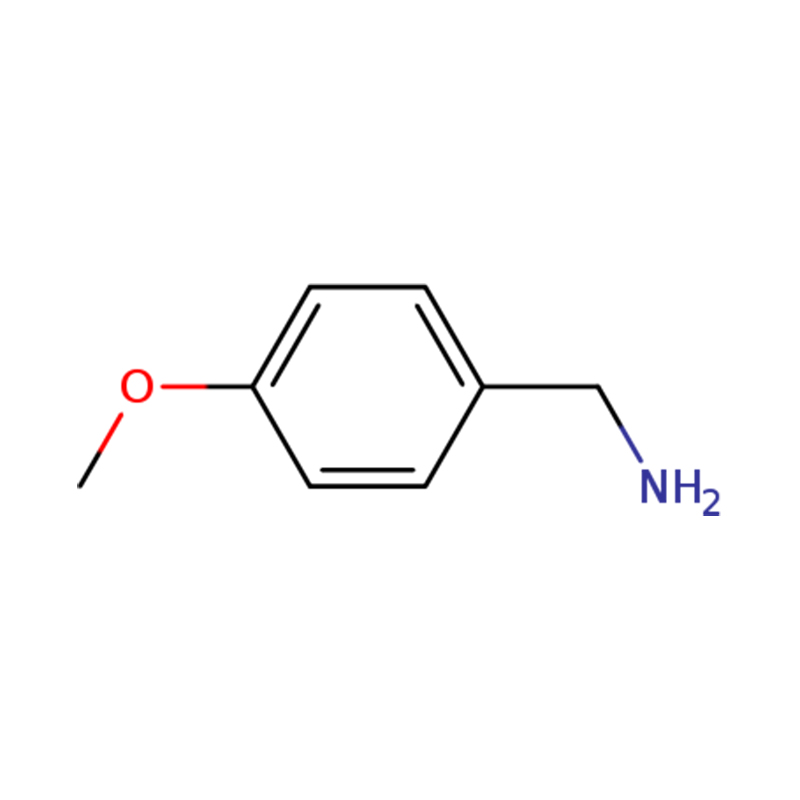
professional factory for Carbetocin Pharmacology - 1-(4-METHOXYPHENYL)METHANAMINE – Gentolex
professional factory for Carbetocin Pharmacology - 1-(4-METHOXYPHENYL)METHANAMINE – Gentolex Detail:
Product Detail
| CasNo | 2393-23-9 | DeliveryTime | within 10 days |
| Molecular | C8H11NO | Production Capacity | 1 Metric Ton/Day |
| Appearance | Clear,colorless to slightly yellow liquid | Purity | 99%min |
| Application | Pharmaceutical intermediates | Storage | Room temperature, dark, sealed |
| LimitNum | 1 Kilogram | Transportation | Air, Sea, Express. |
| Density | 1.05g/mLat25°C(lit.) | Boiling Point | 236-237°C(lit.) |
| Melting Ponit | -10°C | Refractive index | n20/D1.546(lit.) |
| Flash Point: | >230°F | Solubility | Highly soluble in water |
| Name | p-anisylamine or (4-methoxyphenyl)methanamine |
Synonyms
LABOTEST-BB LTBB000703; AKOS BBS-00003589; 4-AMINOMETHYL-ANISOLE; 4-METHOXYBENZYLAMINE; P-Methoxybenzylamine Hydrochloride173.64; 4-Methoxybenzylamine, 98+%; for Sparfloxacine; P-METHOXYBENZYLAMINE HYDROCHLORIDE
Application
It can be used for the synthesis of pharmaceutical intermediates. It is slightly harmful to water. Do not let undiluted or large quantities of products come into contact with groundwater, waterways or sewage systems. Without government permission, do not discharge materials into the surrounding environment to avoid oxides, acids. , air, carbon dioxide contact, keep the container sealed, put it in a tight extractor, and store in a cool, dry place.
QC Lab
An individual QC laboratory stands in the site where chemical, physical test, microbial test, stability study, instrument test such as IR, UV, HPLC, GC are performed for raw materials and finished products. The whole area is access controlled and well maintained with sufficient analytical instruments for intended testing purpose. All instruments are well labelled and appropriately calibrated.
QA
QA is responsible to evaluate and categorize the deviation into Major level, General level and Minor level. For all levels of deviations, the investigation to identify the root cause or potential cause is necessary. Investigation needs to be completed within 7 working days. The product impact assessment along with CAPA plan are also required after the investigation complete and root cause identified. The deviation is closed when the CAPA is implemented. All Level deviation should be approved by QA Manager. After implemented, effectiveness of CAPA is confirmed based on plan.
Product detail pictures:
Related Product Guide:
We generally believe that one's character decides products' top quality, the details decides products' high-quality ,along with the REALISTIC,EFFICIENT AND INNOVATIVE team spirit for professional factory for Carbetocin Pharmacology - 1-(4-METHOXYPHENYL)METHANAMINE – Gentolex , The product will supply to all over the world, such as: Maldives, Singapore, Armenia, We have got constantly insisted on the evolution of solutions, spent good funds and human resource in technological upgrading, and facilitate production improvement, meeting the wants of prospects from all countries and regions.
Good quality and fast delivery, it's very nice. Some products have a little bit problem, but the supplier replaced timely, overall, we are satisfied.