Abstract
Obesity is a chronic, multifactorial metabolic disease associated with substantial morbidity, mortality, and economic burden worldwide. Lifestyle interventions alone often fail to achieve durable weight loss due to complex physiological compensatory mechanisms. Pharmacologic therapies targeting incretin pathways have emerged as effective adjuncts to lifestyle modification. Tirzepatide, a novel dual agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors, has demonstrated promising metabolic effects in patients with type 2 diabetes. This review synthesizes evidence from a large phase 3 randomized controlled trial (SURMOUNT-1) evaluating tirzepatide in adults with obesity or overweight without diabetes. Tirzepatide produced substantial, dose-dependent, and sustained weight loss over 72 weeks, with significant improvements in cardiometabolic risk factors and acceptable safety. These findings position tirzepatide as a potentially transformative therapy in long-term obesity management.
Keywords: Obesity; Tirzepatide; GIP; GLP-1; Weight loss; Cardiometabolic risk
1. Introduction
Obesity is one of the most prevalent chronic diseases globally and represents a major driver of cardiovascular disease, type 2 diabetes mellitus, nonalcoholic fatty liver disease, musculoskeletal disorders, and several malignancies. The World Health Organization estimates that more than 650 million adults worldwide live with obesity, and prevalence continues to rise. Beyond its clinical consequences, obesity imposes a profound economic burden on healthcare systems and society.
Traditional approaches to obesity treatment have emphasized lifestyle modification, including caloric restriction, increased physical activity, and behavioral therapy. While such interventions are foundational, long-term weight maintenance remains challenging. Physiological adaptations such as reductions in resting energy expenditure, increases in hunger-promoting hormones, and enhanced reward-driven eating behavior often counteract weight loss, leading to weight regain. These biological realities underscore the need for effective pharmacologic therapies that target the neuroendocrine mechanisms underlying energy balance.
Incretin-based therapies have emerged as a major advance in metabolic disease management. Glucagon-like peptide-1 (GLP-1) receptor agonists have demonstrated significant weight loss and cardiometabolic benefits. More recently, glucose-dependent insulinotropic polypeptide (GIP) has been recognized as an important regulator of energy metabolism. Tirzepatide, a first-in-class dual GIP and GLP-1 receptor agonist, integrates the actions of both incretins into a single molecule, offering the potential for superior weight loss efficacy. This article provides a comprehensive synthesis of the efficacy, safety, and clinical implications of tirzepatide in the treatment of obesity, based primarily on findings from the SURMOUNT-1 trial.
2. Pharmacological Mechanisms of Tirzepatide
Tirzepatide is a synthetic peptide engineered from the native GIP sequence with additional agonist activity at the GLP-1 receptor. It exhibits high affinity for the GIP receptor and moderate affinity for the GLP-1 receptor, allowing it to engage complementary metabolic pathways.
2.1 Central Appetite Regulation
Tirzepatide activates appetite-regulating centers in the hypothalamus, enhancing satiety signals and suppressing hunger. This central mechanism contributes significantly to reduced caloric intake and sustained weight loss.
2.2 Gastrointestinal Motility
The drug slows gastric emptying, prolonging postprandial fullness and attenuating rapid glucose absorption. This effect supports appetite control and postprandial glycemic regulation.
2.3 Glucose Homeostasis and Insulin Sensitivity
By stimulating glucose-dependent insulin secretion and suppressing glucagon release, tirzepatide improves glycemic control and reduces insulin resistance. These effects are particularly relevant for individuals with prediabetes or metabolic syndrome.
2.4 Adipose Tissue and Energy Metabolism
Dual GIP and GLP-1 receptor activation influences adipose tissue metabolism by promoting lipolysis and inhibiting lipogenesis. This contributes to preferential fat mass reduction while preserving lean body mass, thereby improving overall body composition.
3. Study Design and Methodology
The SURMOUNT-1 trial was a multinational, randomized, double-blind, placebo-controlled phase 3 study designed to evaluate the efficacy and safety of tirzepatide in adults with obesity or overweight without diabetes.
3.1 Participants
Eligible participants were adults aged 18 years or older with a body mass index (BMI) ≥30 kg/m², or ≥27 kg/m² with at least one weight-related comorbidity (e.g., hypertension, dyslipidemia, obstructive sleep apnea). Individuals with diabetes, recent weight changes, prior bariatric surgery, or recent use of weight-loss medications were excluded.
3.2 Interventions
Participants were randomized in a 1:1:1:1 ratio to receive once-weekly subcutaneous injections of tirzepatide at doses of 5 mg, 10 mg, or 15 mg, or placebo, for 72 weeks. A dose-escalation phase lasting up to 20 weeks was implemented to enhance tolerability. All participants received standardized lifestyle counseling, including dietary caloric reduction (approximately 500 kcal/day deficit) and recommendations for at least 150 minutes of moderate physical activity per week.
3.3 Outcomes
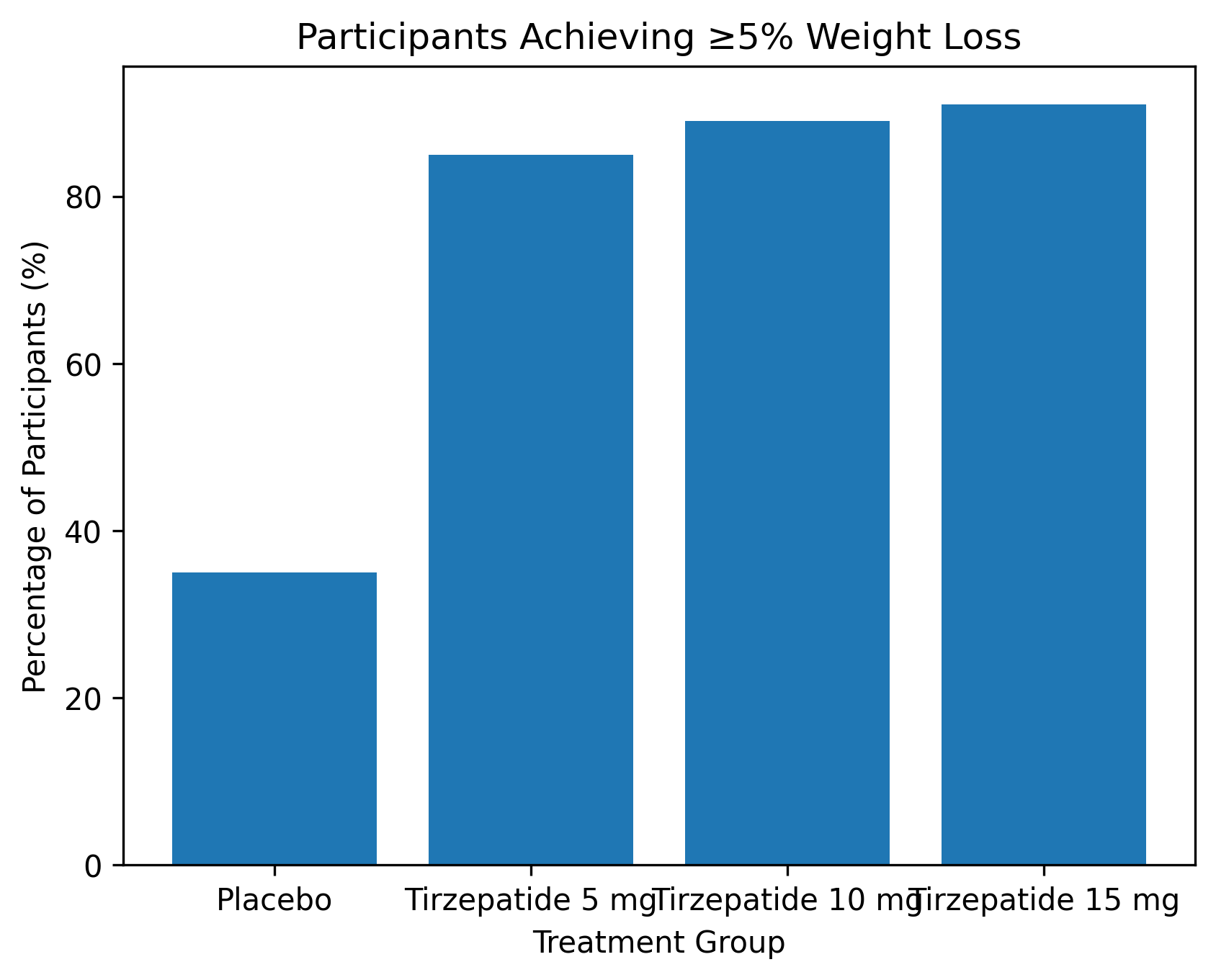
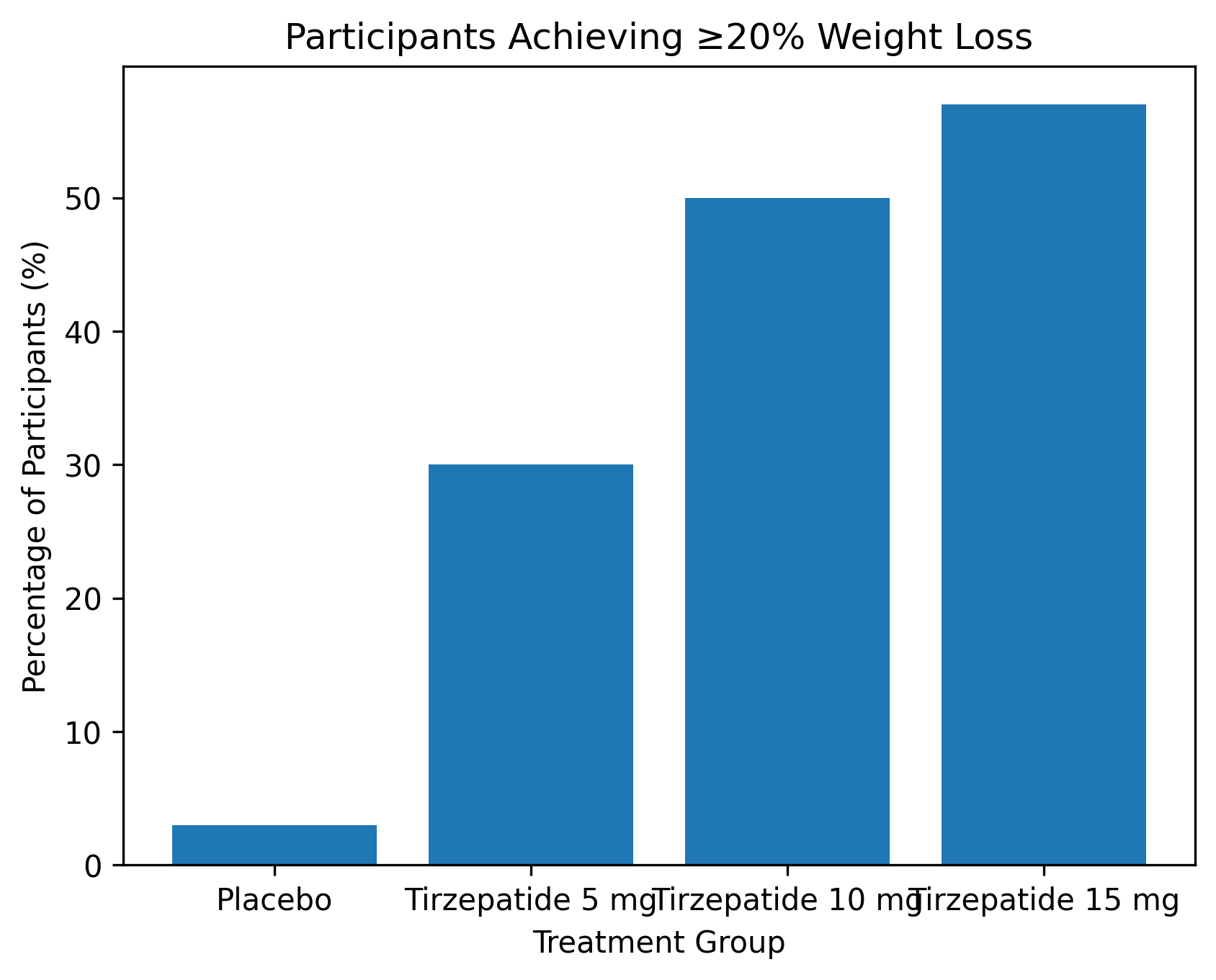
The coprimary endpoints were the percentage change in body weight from baseline to week 72 and the proportion of participants achieving at least 5% weight loss. Key secondary endpoints included proportions achieving ≥10%, ≥15%, and ≥20% weight loss, changes in waist circumference, blood pressure, lipid levels, insulin sensitivity, body composition, and health-related quality of life.
4. Efficacy Outcomes
4.1 Weight Reduction
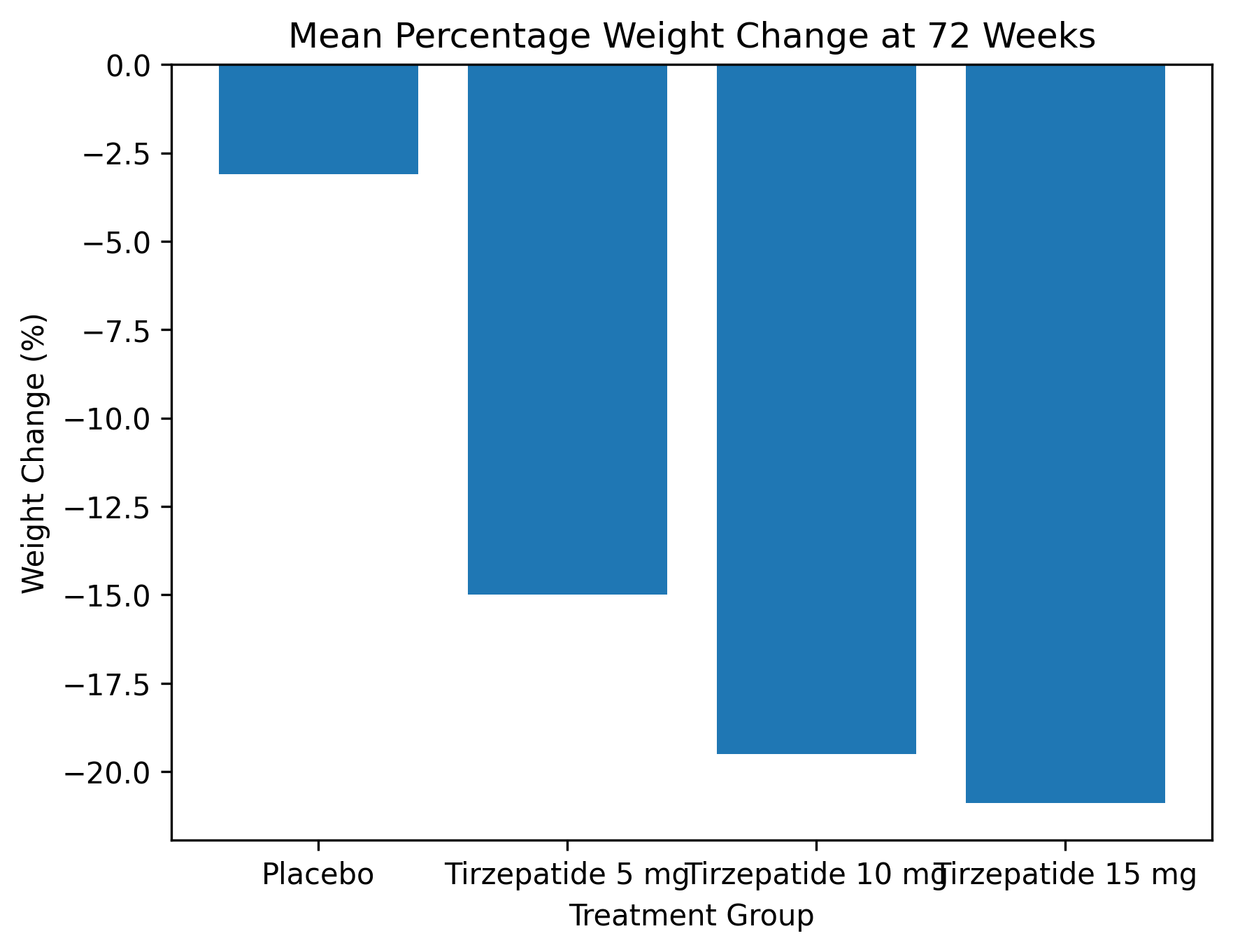
Tirzepatide produced substantial, dose-dependent, and sustained weight loss over the 72-week treatment period. Mean percentage reductions in body weight were approximately:
1. 15% with 5 mg,
2. 19.5% with 10 mg,
3. 20.9% with 15 mg,
compared with approximately 3% in the placebo group.
These reductions exceed those typically observed with older anti-obesity medications and approach the magnitude of weight loss seen with certain metabolic surgical procedures.
4.2 Achievement of Clinically Meaningful Weight Loss
A large majority of participants receiving tirzepatide achieved clinically meaningful weight loss. More than 85% of participants in the active treatment groups achieved ≥5% weight loss, and over half of those receiving 10 mg or 15 mg achieved ≥20% weight loss. These findings highlight tirzepatide’s potential to meet or exceed weight loss thresholds associated with meaningful improvements in metabolic health.
4.3 Body Composition
Dual-energy X-ray absorptiometry analyses demonstrated that weight loss with tirzepatide was driven predominantly by reductions in fat mass, with a relatively smaller decrease in lean mass. This favorable fat-to-lean mass ratio is consistent with healthy weight loss and may help preserve functional capacity and resting metabolic rate.
5. Effects on Cardiometabolic Risk Factors
Beyond weight reduction, tirzepatide conferred broad metabolic benefits:
5.1 Waist Circumference and Visceral Adiposity
Significant reductions in waist circumference were observed, suggesting a decrease in visceral adiposity, which is strongly linked to cardiometabolic risk.
5.2 Blood Pressure
Modest but clinically meaningful reductions in both systolic and diastolic blood pressure occurred, potentially contributing to long-term cardiovascular risk reduction.
5.3 Lipid Profile
Tirzepatide improved lipid parameters, including reductions in triglycerides and low-density lipoprotein cholesterol and increases in high-density lipoprotein cholesterol.
5.4 Glycemic Control and Prediabetes Reversal
Marked improvements in insulin sensitivity and fasting insulin levels were observed. Among participants with prediabetes at baseline, more than 95% reverted to normoglycemia after treatment, compared with approximately 62% in the placebo group.
5.5 Health-Related Quality of Life
Participants receiving tirzepatide reported greater improvements in physical functioning and overall health-related quality of life, reflecting not only metabolic but also functional and psychosocial benefits.
6. Safety and Tolerability
6.1 Common Adverse Events
The most frequently reported adverse events were gastrointestinal, including nausea, diarrhea, vomiting, and constipation. These events were generally mild to moderate in severity, occurred primarily during the dose-escalation phase, and tended to diminish over time.
6.2 Serious Adverse Events
The incidence of serious adverse events was similar across tirzepatide and placebo groups. No clear signal emerged for severe pancreatitis, medullary thyroid carcinoma, or other rare but serious adverse outcomes.
6.3 Treatment Discontinuation
Discontinuation due to adverse events occurred slightly more frequently in the tirzepatide groups than in the placebo group but remained within acceptable limits, supporting the overall tolerability of the therapy.
7. Comparison with Existing Obesity Treatments
Compared with traditional anti-obesity medications, tirzepatide demonstrates superior efficacy in both the magnitude and durability of weight loss. While GLP-1 receptor agonists such as semaglutide have already set a high benchmark for pharmacologic weight loss, tirzepatide appears to achieve even greater average reductions, likely due to the synergistic effects of dual GIP and GLP-1 receptor activation.
Notably, the degree of weight loss observed with tirzepatide approaches that achieved with some forms of bariatric surgery, without the risks associated with invasive procedures. This positions tirzepatide as a potential alternative for patients who are unwilling or unsuitable candidates for surgical intervention.
8. Clinical Implications and Future Directions
The findings from SURMOUNT-1 support a paradigm shift in obesity management, recognizing obesity as a chronic disease requiring long-term, biologically targeted therapy. Tirzepatide offers clinicians a powerful tool to achieve substantial and sustained weight loss, improve cardiometabolic risk profiles, and enhance patient quality of life.
Future research should focus on:
1. Long-term cardiovascular and renal outcomes associated with tirzepatide-induced weight loss.
2. Durability of weight loss following treatment discontinuation.
3. Optimal dosing strategies and individualized treatment selection.
4. Efficacy and safety in special populations, including older adults, adolescents, and individuals with multiple comorbidities.
9. Limitations
Despite the strengths of the trial, certain limitations warrant consideration. Participants may have been more motivated than the general population, potentially limiting generalizability. Baseline cardiometabolic risk factors were relatively well controlled, which may have attenuated observable improvements. Additionally, the proportion of participants with overweight but not obesity was small, limiting conclusions for this subgroup.
10. Conclusion
Once-weekly tirzepatide produces substantial, sustained, and clinically meaningful weight loss in adults with obesity or overweight without diabetes, accompanied by broad improvements in cardiometabolic health and acceptable safety. Its dual incretin mechanism represents a significant advancement in obesity pharmacotherapy. Tirzepatide has the potential to redefine the standard of care for obesity and serve as a cornerstone of long-term, comprehensive weight management strategies.
Tirzepatide represents a significant advancement in the treatment of metabolic conditions, offering dual benefits for weight management and glycemic control. This innovative medication functions through a unique dual-agonist mechanism, simultaneously activating both GLP-1 and GIP receptors. This combined action produces enhanced therapeutic effects including appetite suppression, improved insulin sensitivity, and promoted weight reduction, making it particularly valuable for individuals managing obesity or Type 2 diabetes.
The good news is that taking a short break from tirzepatide (for a week or so) usually won't cause any significant issues. Your doctor may recommend alternative ways to manage your blood sugar or appetite during the break, but most people can resume tirzepatide without any major setbacks once they're ready.
Tirzepatide promotes weight loss through its innovative dual-agonist action, which engages both the GIP and GLP-1 hormone receptors. This coordinated activity produces a synergistic effect that targets multiple physiological processes involved in weight regulation.
By mimicking these natural incretin hormones, the medication effectively suppresses appetite and enhances feelings of satiety, leading to a natural reduction in caloric consumption. Concurrently, it optimizes metabolic function by improving insulin sensitivity and supporting healthier blood glucose levels.
The comprehensive nature of this dual-receptor activation facilitates substantial and sustained weight reduction. For optimal outcomes, this pharmaceutical intervention is most effective when integrated with foundational lifestyle measures, including a balanced diet and regular physical activity, creating a holistic approach to long-term weight management and metabolic health improvement.
Tirzepatide is generally considered safe for most people when prescribed and monitored by a healthcare professional, but—like any medication—it comes with potential side effects and risks that should be understood before starting treatment.
Tirzepatide and Semaglutide are both injectable medications for type 2 diabetes and obesity, but they work differently: Semaglutide is a GLP-1 receptor agonist, while Tirzepatide is a dual GIP/GLP-1 receptor agonist. Overall, Tirzepatide generally provides greater blood sugar reduction and weight loss but may cause slightly more gastrointestinal side effects. Semaglutide has a longer track record with more established long-term safety data. Choice depends on individual goals: Tirzepatide may be preferred for maximum weight loss and strong glucose control, while Semaglutide may be better for tolerability and long-term safety.
Post time: Jan-21-2026