Background
Incretin-based therapies have long been known to improve both blood glucose control and body weight reduction. Traditional incretin drugs primarily target the GLP-1 receptor, while Tirzepatide represents a new generation of “twincretin” agents — acting on both GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 receptors.
This dual action has been shown to enhance metabolic benefits and promote greater weight loss compared to GLP-1 agonists alone.
The SURMOUNT-1 Study Design
SURMOUNT-1 was a randomized, double-blind, phase 3 clinical trial conducted across 119 sites in nine countries.
Participants included adults who were:
- Obese (BMI ≥ 30), or
- Overweight (BMI ≥ 27) with at least one weight-related comorbidity (e.g., hypertension, dyslipidemia, sleep apnea, or cardiovascular disease).
Individuals with diabetes, recent weight-loss drug use, or prior bariatric surgery were excluded.
Participants were randomly assigned to receive once-weekly injections of:
- Tirzepatide 5 mg, 10 mg, 15 mg, or
- Placebo
All participants also received lifestyle guidance:
- A caloric deficit of 500 kcal/day
- At least 150 minutes of physical activity per week
The treatment lasted 72 weeks, including a 20-week dose-escalation phase followed by a 52-week maintenance period.
Results Overview
A total of 2,359 participants were enrolled.
The average age was 44.9 years, 67.5% were women, with a mean body weight of 104.8 kg and BMI of 38.0.
Mean Body Weight Reduction at Week 72
| Dose Group | % Weight Change | Mean Weight Change (kg) | Additional Loss vs Placebo |
|---|---|---|---|
| 5 mg | -15.0% | -16.1 kg | -13.5% |
| 10 mg | -19.5% | -22.2 kg | -18.9% |
| 15 mg | -20.9% | -23.6 kg | -20.1% |
| Placebo | -3.1% | -2.4 kg | — |
Tirzepatide achieved 15–21% mean body weight reduction, demonstrating clear dose-dependent effects.
Percentage of Participants Achieving Target Weight Loss
| Weight Loss (%) | 5 mg | 10 mg | 15 mg | Placebo |
|---|---|---|---|---|
| ≥5% | 85.1% | 88.9% | 90.9% | 34.5% |
| ≥10% | 68.5% | 78.1% | 83.5% | 18.8% |
| ≥15% | 48.0% | 66.6% | 70.6% | 8.8% |
| ≥20% | 30.0% | 50.1% | 56.7% | 3.1% |
| ≥25% | 15.3% | 32.3% | 36.2% | 1.5% |
More than half of participants receiving ≥10 mg Tirzepatide achieved ≥20% weight loss, approaching the effect seen with bariatric surgery.
Metabolic and Cardiovascular Benefits
Compared with placebo, Tirzepatide significantly improved:
- Waist circumference
- Systolic blood pressure
- Lipid profile
- Fasting insulin levels
Among participants with prediabetes, 95.3% returned to normal glucose levels, compared to 61.9% in the placebo group — indicating Tirzepatide not only aids in weight reduction but also improves glucose metabolism.
Safety and Tolerability
The most common side effects were gastrointestinal, including nausea, diarrhea, and constipation, mostly mild and transient.
The discontinuation rate due to adverse events was approximately 4–7%.
A few deaths occurred during the trial, primarily linked to COVID-19, and were not directly related to the study drug.
No significant differences were observed in gallbladder-related complications.
Discussion
Lifestyle modification alone (diet and exercise) typically produces only ~3% average weight loss, as seen in the placebo group.
In contrast, Tirzepatide enabled 15–21% total body weight reduction, representing a 5–7 times greater effect.
Compared with:
- Oral weight-loss drugs: usually achieve 5–10% loss
- Bariatric surgery: achieves >20% loss
Tirzepatide bridges the gap between pharmacologic and surgical interventions — offering powerful, non-invasive weight reduction.
Importantly, concerns about worsening glucose metabolism were not observed. On the contrary, Tirzepatide improved insulin sensitivity and reversed prediabetes in most participants.
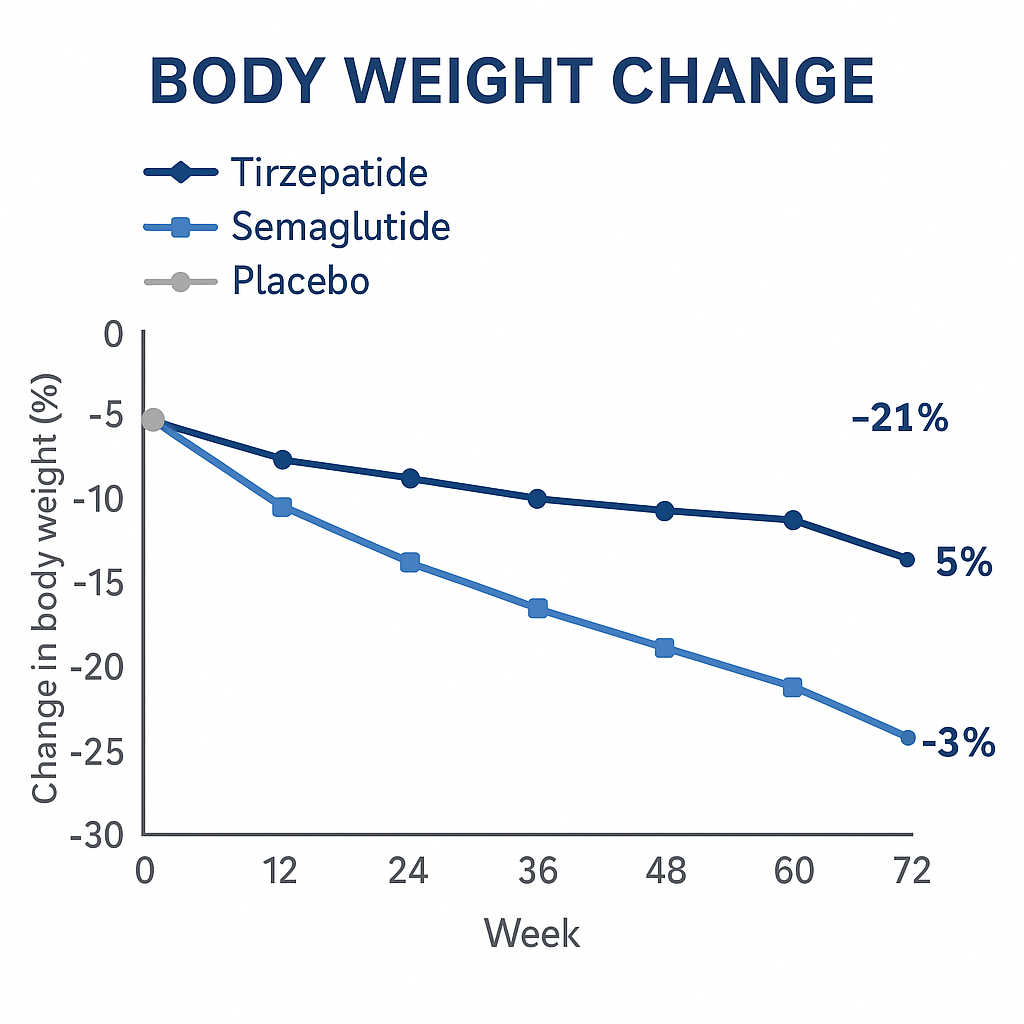
However, this trial compared Tirzepatide with placebo — not directly with Semaglutide.
A head-to-head comparison is needed to determine which agent produces greater weight loss.
Conclusion
For adults with obesity or overweight and related comorbidities, adding once-weekly Tirzepatide to a structured lifestyle program (diet + exercise) can lead to:
- 15–21% average body weight reduction
- Significant metabolic improvements
- High tolerability and safety
Tirzepatide thus represents an effective and clinically validated therapy for sustainable, medically supervised weight management.
Post time: Oct-16-2025