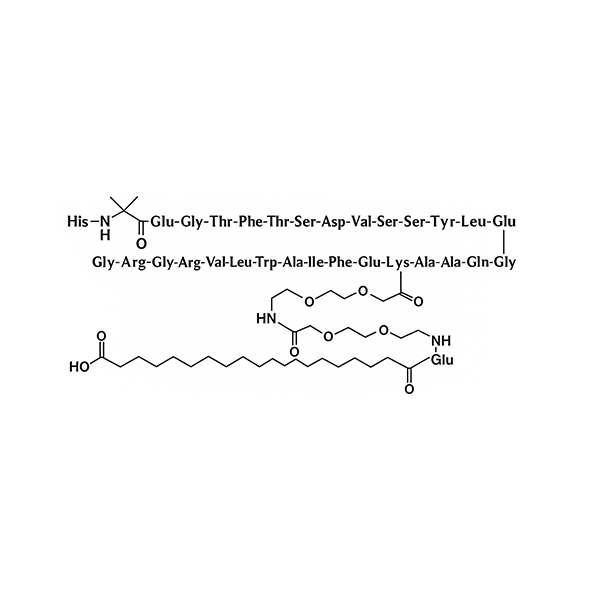
Semaglutide API
Semaglutide is a potent glucagon-like peptide-1 (GLP-1) receptor agonist with approximately 94% amino acid sequence homology to human GLP-1. By activating the GLP-1 receptor, Semaglutide stimulates insulin secretion, suppresses glucagon release, delays gastric emptying, and increases satiety, making it an effective therapeutic agent for the treatment of type 2 diabetes mellitus and obesity. As a long-acting GLP-1 receptor agonist, Semaglutide demonstrates superior glycemic control, sustained efficacy, and improved patient compliance compared with conventional therapies.
The prolonged pharmacological activity of Semaglutide is primarily attributed to its structural modifications. The incorporation of α-aminoisobutyric acid (Aib) enhances molecular stability and reduces enzymatic degradation. In addition, the attachment of a fatty acid side chain enables strong binding to serum albumin, significantly increasing molecular weight and extending the plasma half-life. These modifications allow for reduced dosing frequency and improved therapeutic outcomes.
Pharmacokinetics and Metabolism
Semaglutide is primarily metabolized via β-oxidation of its fatty acid side chain. The metabolites are eliminated mainly through feces and urine, with a small fraction of the unchanged drug excreted in urine. Semaglutide exhibits a favorable safety and tolerability profile. The most commonly reported adverse reactions are gastrointestinal events, including nausea, vomiting, diarrhea, and constipation, which are generally mild to moderate and tend to resolve over time. Overall, Semaglutide offers a high level of clinical safety and patient acceptance.
Synthesis of Semaglutide API
The production of Semaglutide API is typically achieved through solid-phase peptide synthesis (SPPS). A specialized solid-phase reactor is employed to enable stepwise amino acid coupling with high precision. Advanced reactor systems, consisting of a reactor shell, gas inlet and outlet pipelines, gas distribution components, and solid-phase catalytic components, provide improved reaction uniformity and efficiency.
During Semaglutide synthesis, these optimized reactors are particularly suitable for solid-phase catalytic reactions, resulting in enhanced reaction performance, reduced processing time, and higher product purity. Compared with conventional equipment, this process reduces overall reaction time by approximately 20%–30% and lowers total production costs by about 10%. The method is robust, scalable, and well-suited for industrial manufacturing, supporting the further research, development, and commercialization of Semaglutide.
Purification of Semaglutide API
Crude Semaglutide obtained from solid-phase synthesis contains various impurities, including deletion peptides, truncated sequences, isomers, and residual reagents, which adversely affect purity, safety, and yield. Therefore, efficient purification is a critical step in API production.
State-of-the-art peptide purification technologies, including preparative high-performance liquid chromatography (Prep-HPLC) and multi-dimensional purification strategies, enable the final product purity to exceed 99%, with individual impurities controlled below 0.2%. In addition to achieving high purity, these advanced purification methods significantly improve the solubility and stability of the final product. Sample recovery rates can reach up to 100%, and the process is stable, reproducible, and suitable for large-scale industrial production, effectively meeting both scientific research and commercial manufacturing requirements.
Conclusion
Semaglutide API is a highly effective, long-acting GLP-1 receptor agonist with significant clinical value in the treatment of type 2 diabetes and obesity. Through rational molecular design, advanced solid-phase synthesis, and high-efficiency purification technologies, the API achieves superior purity, stability, solubility, and cost-effectiveness. The mature and reliable production process supports both research and large-scale commercial supply, ensuring high-quality availability for pharmaceutical development.
Post time: Jan-29-2026