1. Product Overview
Retatrutide is a novel long-acting peptide drug and a GLP-1/GIP/Glucagon triple receptor agonist, primarily developed for research in obesity, type 2 diabetes, and related metabolic disorders. Its unique triple-target mechanism offers significant advantages in weight management and metabolic regulation.
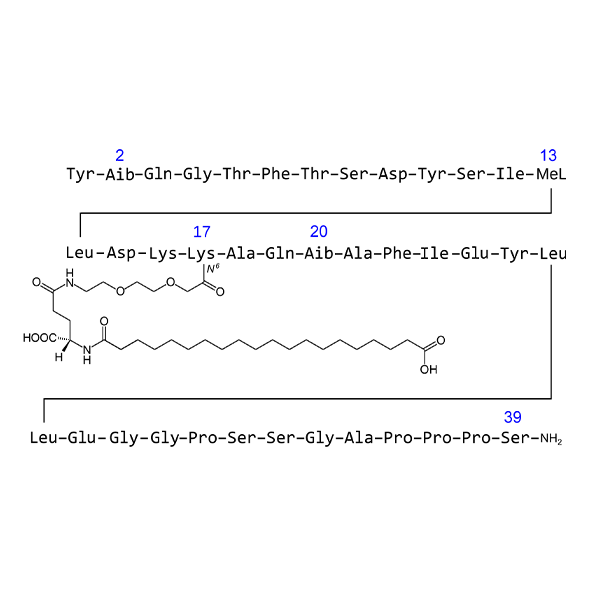
As a key upstream substance, Retatrutide API (Active Pharmaceutical Ingredient) is essential for drug formulation, preclinical and clinical studies, and eventual commercialization.
2. Primary Research and Applications
Retatrutide API is mainly used in the following research areas:
-
Obesity and Weight Management: Promotes significant weight loss and improves body fat distribution.
-
Type 2 Diabetes: Enhances glycemic control and supports pancreatic β-cell function.
-
Metabolic Syndrome: Regulates blood lipids, glucose, and energy metabolism.
-
Non-Alcoholic Fatty Liver Disease (NAFLD/NASH): Reduces hepatic fat accumulation and improves inflammation.
-
Cardiometabolic Risk Research: Addresses multiple metabolic risk factors simultaneously.
3. Mechanism of Action
Retatrutide activates three receptors simultaneously:
-
GLP-1 receptor: Stimulates insulin secretion, delays gastric emptying, and increases satiety.
-
GIP receptor: Enhances pancreatic function and synergistically regulates blood glucose.
-
Glucagon receptor: Promotes energy expenditure and fat oxidation.
This triple mechanism produces synergistic effects, offering stronger and longer-lasting metabolic benefits compared to single- or dual-target therapies.
4. API Technical Features
Molecular Characteristics
-
Long-acting peptide modified with fatty acid side chains.
-
Requires high precision in synthesis and strict structural consistency.
Manufacturing Process
-
Solid-phase peptide synthesis (SPPS).
-
Multi-step purification (e.g., RP-HPLC) to ensure high purity and low impurity levels.
Quality Control (Typical Parameters)
-
Purity: ≥98% (HPLC)
-
Moisture content: ≤5.0%
-
Endotoxin: Compliant with injectable-grade standards
-
Sequence verification: LC-MS, amino acid sequence analysis
5. Compliance and Supply Capabilities
Retatrutide API for research and clinical applications must meet:
-
GMP or equivalent manufacturing conditions.
-
Complete documentation (COA, MSDS, stability data).
-
Support for DMF (Drug Master File) submission.
-
Batch-to-batch consistency and traceability.
6. Market Value and Development Prospects
With the global prevalence of obesity and diabetes continuing to rise, demand for safe and effective metabolic therapies is increasing. As a next-generation triple-target metabolic drug, Retatrutide API has high technical barriers and broad market potential, positioning it as a key player in the future of metabolic disease research and drug development.
Post time: Feb-06-2026