In recent years, the treatment of obesity and type 2 diabetes has undergone revolutionary progress. Following GLP-1 receptor agonists (e.g., Semaglutide) and dual agonists (e.g., Tirzepatide), Retatrutide (LY3437943), a triple agonist (GLP-1, GIP, and glucagon receptors), has shown unprecedented efficacy. With remarkable results in weight reduction and metabolic improvement, it is regarded as a potential breakthrough therapy for metabolic diseases.
Mechanism of Action
-
GLP-1 receptor activation: Enhances insulin secretion, suppresses appetite, delays gastric emptying.
-
GIP receptor activation: Boosts the glucose-lowering effects of GLP-1, improves insulin sensitivity.
-
Glucagon receptor activation: Promotes energy expenditure and fat metabolism.
The synergy of these three receptors allows Retatrutide to surpass existing drugs in both weight loss and glycemic control.
Clinical Trial Data (Phase II)
In a Phase II trial with 338 overweight/obese patients, Retatrutide demonstrated highly promising outcomes.
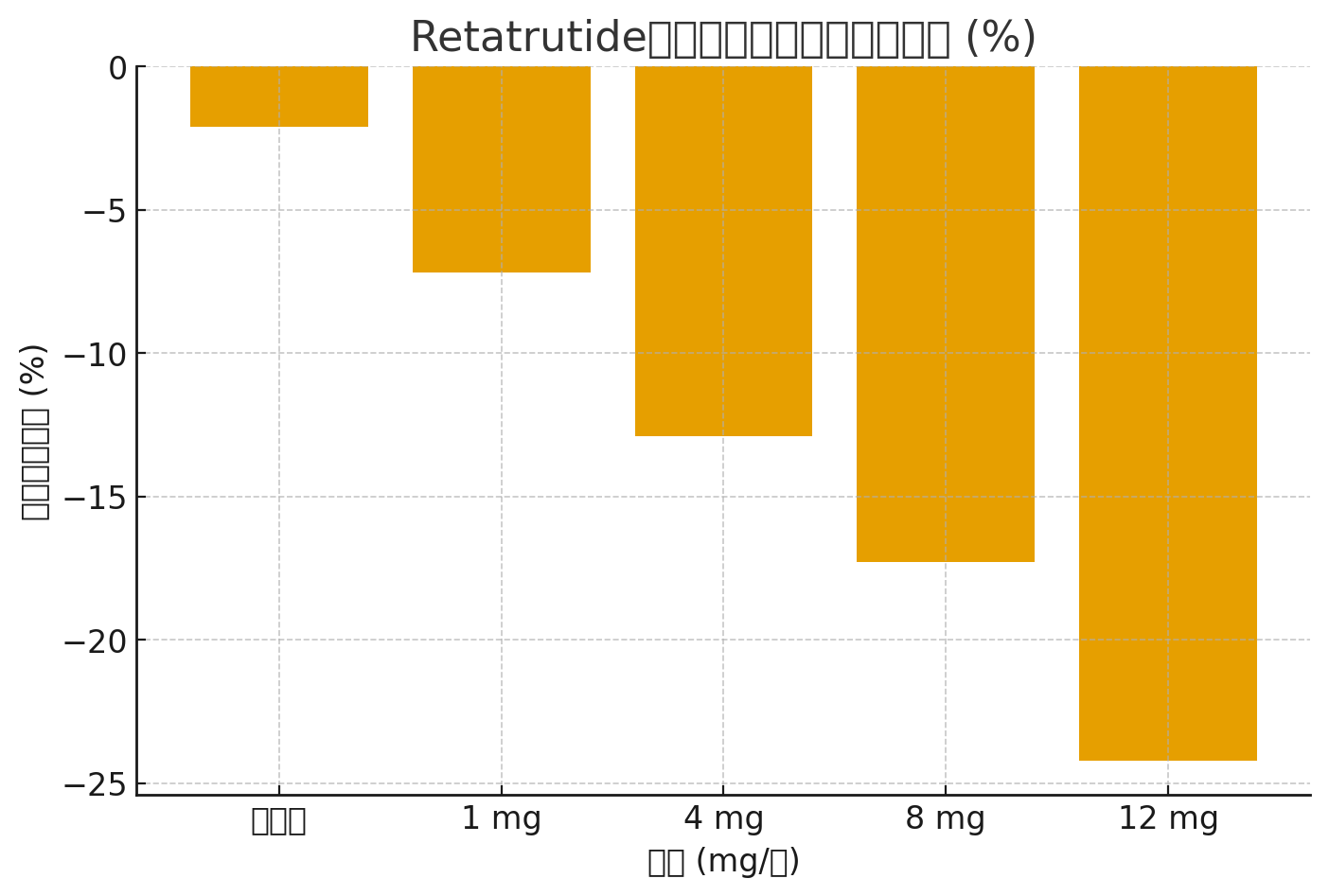
Table: Comparison of Retatrutide vs. Placebo
| Dose (mg/week) | Mean Weight Reduction (%) | HbA1c Reduction (%) | Common Adverse Events |
|---|---|---|---|
| 1 mg | -7.2% | -0.9% | Nausea, mild vomiting |
| 4 mg | -12.9% | -1.5% | Nausea, appetite loss |
| 8 mg | -17.3% | -2.0% | GI discomfort, mild diarrhea |
| 12 mg | -24.2% | -2.2% | Nausea, appetite loss, constipation |
| Placebo | -2.1% | -0.2% | No significant change |
Data Visualization (Weight Reduction Comparison)
The following bar chart illustrates the average weight reduction across different Retatrutide doses compared with placebo:
Post time: Sep-16-2025