Background and Study Design
Retatrutide (LY3437943) is a novel single-peptide drug that activates three receptors simultaneously: GIP, GLP-1, and glucagon. To evaluate its efficacy and safety in individuals with obesity but without diabetes, a phase 2, randomized, double-blind, placebo-controlled trial was conducted (NCT04881760). A total of 338 participants with a BMI ≥30, or ≥27 with at least one weight-related comorbidity, were randomized to receive placebo or retatrutide (1 mg, 4 mg with two titration schedules, 8 mg with two titration schedules, or 12 mg) administered once weekly by subcutaneous injection for 48 weeks. The primary endpoint was the percentage change in body weight at 24 weeks, with secondary endpoints including weight change at 48 weeks and categorical weight-loss thresholds (≥5%, ≥10%, ≥15%).
Key Results
-
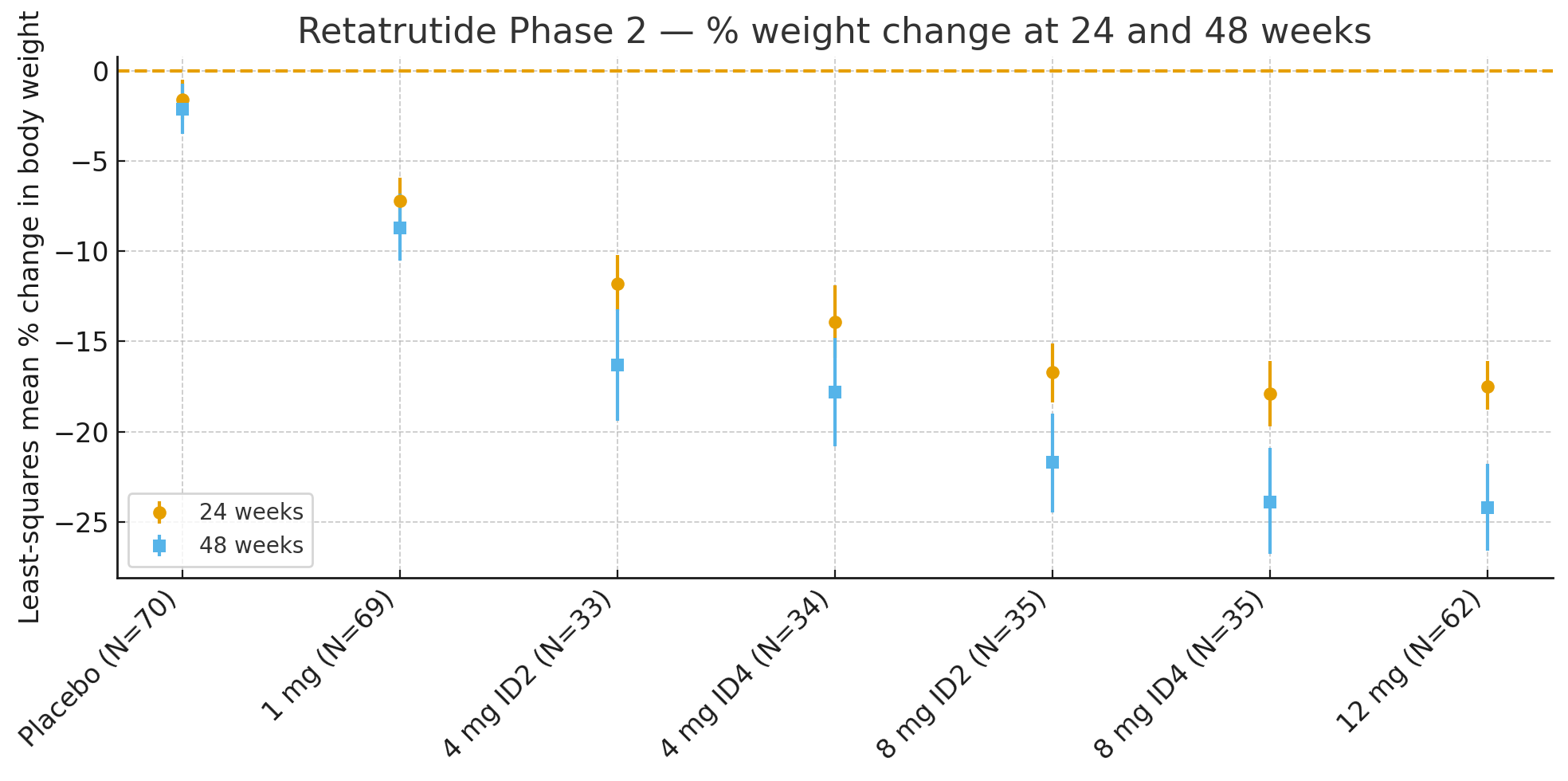
24 weeks: Least-squares mean percent change in body weight relative to baseline was
-
Placebo: −1.6%
-
1 mg: −7.2%
-
4 mg (combined): −12.9%
-
8 mg (combined): −17.3%
-
12 mg: −17.5%
-
-
48 weeks: Percent change in body weight was
-
Placebo: −2.1%
-
1 mg: −8.7%
-
4 mg (combined): −17.1%
-
8 mg (combined): −22.8%
-
12 mg: −24.2%
-
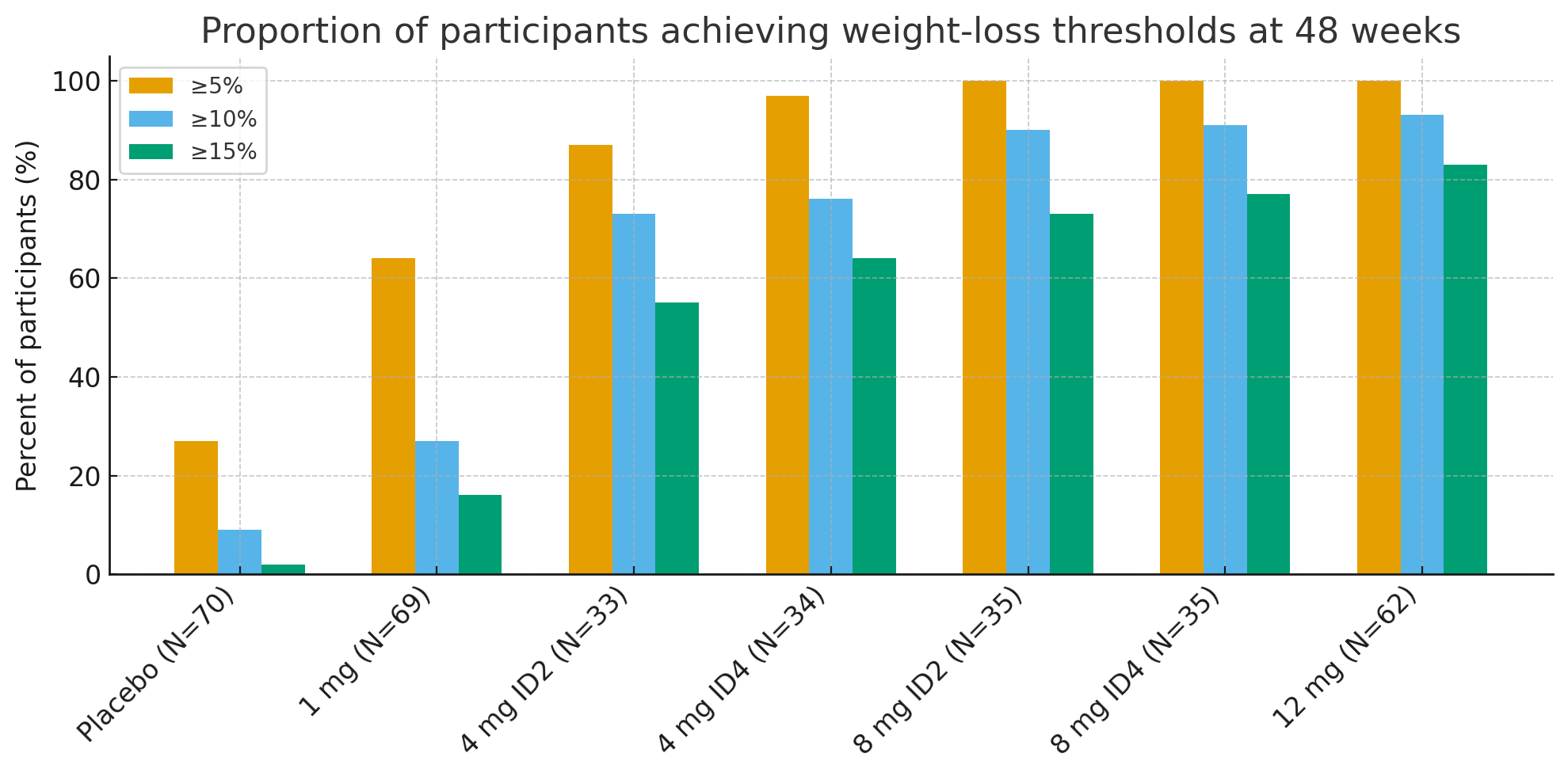
At 48 weeks, the proportions of participants achieving clinically meaningful weight-loss thresholds were striking:
-
≥5% weight loss: 27% with placebo vs. 92–100% in active groups
-
≥10%: 9% with placebo vs. 73–93% in active groups
-
≥15%: 2% with placebo vs. 55–83% in active groups
In the 12 mg group, up to 26% of participants lost ≥30% of their baseline weight, a magnitude of weight loss comparable to bariatric surgery.
Safety
The most common adverse events were gastrointestinal (nausea, vomiting, diarrhea), generally mild to moderate and dose-related. Lower starting doses (2 mg titration) reduced these events. Dose-related increases in heart rate were observed, peaking at week 24, then declining. Discontinuation rates ranged from 6–16% across active groups, somewhat higher than placebo.
Conclusions
In adults with obesity without diabetes, weekly subcutaneous retatrutide for 48 weeks produced substantial, dose-dependent reductions in body weight (up to ~24% mean loss at the highest dose), along with improvements in cardiometabolic markers. Gastrointestinal adverse events were frequent but manageable with titration. These phase 2 findings suggest retatrutide could represent a new therapeutic benchmark for obesity, pending confirmation in larger, long-term phase 3 trials.
Post time: Sep-28-2025