High Quality for Cas 2199-59-9 With Purity 99% - 1-(4-METHOXYPHENYL)METHANAMINE – Gentolex
High Quality for Cas 2199-59-9 With Purity 99% - 1-(4-METHOXYPHENYL)METHANAMINE – Gentolex Detail:
Product Detail
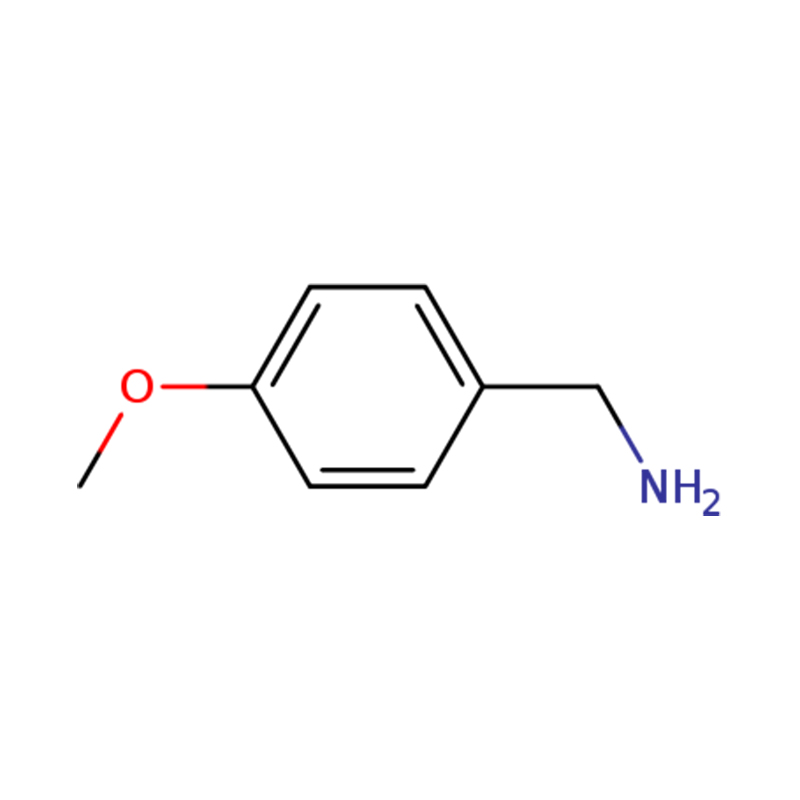
| CasNo | 2393-23-9 | DeliveryTime | within 10 days |
| Molecular | C8H11NO | Production Capacity | 1 Metric Ton/Day |
| Appearance | Clear,colorless to slightly yellow liquid | Purity | 99%min |
| Application | Pharmaceutical intermediates | Storage | Room temperature, dark, sealed |
| LimitNum | 1 Kilogram | Transportation | Air, Sea, Express. |
| Density | 1.05g/mLat25°C(lit.) | Boiling Point | 236-237°C(lit.) |
| Melting Ponit | -10°C | Refractive index | n20/D1.546(lit.) |
| Flash Point: | >230°F | Solubility | Highly soluble in water |
| Name | p-anisylamine or (4-methoxyphenyl)methanamine |
Synonyms
LABOTEST-BB LTBB000703; AKOS BBS-00003589; 4-AMINOMETHYL-ANISOLE; 4-METHOXYBENZYLAMINE; P-Methoxybenzylamine Hydrochloride173.64; 4-Methoxybenzylamine, 98+%; for Sparfloxacine; P-METHOXYBENZYLAMINE HYDROCHLORIDE
Application
It can be used for the synthesis of pharmaceutical intermediates. It is slightly harmful to water. Do not let undiluted or large quantities of products come into contact with groundwater, waterways or sewage systems. Without government permission, do not discharge materials into the surrounding environment to avoid oxides, acids. , air, carbon dioxide contact, keep the container sealed, put it in a tight extractor, and store in a cool, dry place.
QC Lab
An individual QC laboratory stands in the site where chemical, physical test, microbial test, stability study, instrument test such as IR, UV, HPLC, GC are performed for raw materials and finished products. The whole area is access controlled and well maintained with sufficient analytical instruments for intended testing purpose. All instruments are well labelled and appropriately calibrated.
QA
QA is responsible to evaluate and categorize the deviation into Major level, General level and Minor level. For all levels of deviations, the investigation to identify the root cause or potential cause is necessary. Investigation needs to be completed within 7 working days. The product impact assessment along with CAPA plan are also required after the investigation complete and root cause identified. The deviation is closed when the CAPA is implemented. All Level deviation should be approved by QA Manager. After implemented, effectiveness of CAPA is confirmed based on plan.
Product detail pictures:
Related Product Guide:
To create more value for customers is our business philosophy; customer growing is our working chase for High Quality for Cas 2199-59-9 With Purity 99% - 1-(4-METHOXYPHENYL)METHANAMINE – Gentolex , The product will supply to all over the world, such as: Kuwait, Bogota, Serbia, There are advanced producing & processing equipment and skilled workers to ensure the products with high quality. We have found an excellent before-sale, sale, after-sale service to ensure the customers that could rest assured to make orders. Until now our products are now moving on fast and very popular in South America, East Asia, the Middle east, Africa, etc.
Customer service staff and sales man are very patience and they all good at English, product's arrival is also very timely, a good supplier.