Baloxavir Marboxil Manufacturer in China: Quality & Reliability Guaranteed
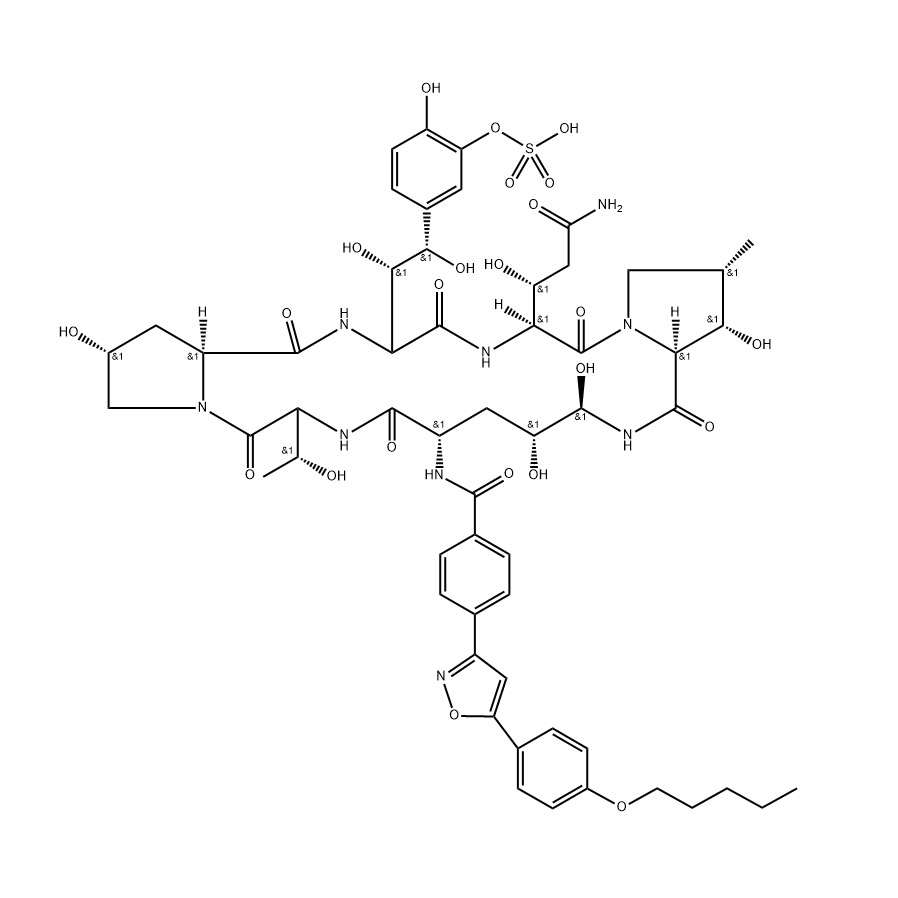
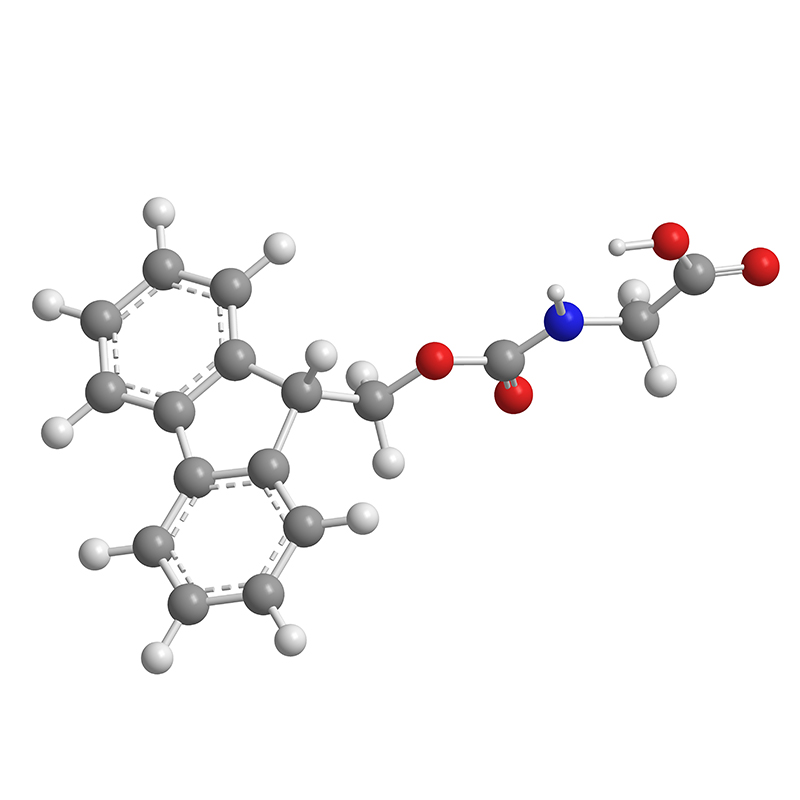
As a leading manufacturer in China, we proudly offer Baloxavir Marboxil, an innovative antiviral agent designed to combat influenza effectively. My personal experience with this product has been nothing short of impressive, as it efficiently inhibits viral replication and shortens the duration of illness. I know how crucial it is for B2B purchasers to find reliable suppliers, and that's why our manufacturing processes adhere strictly to international quality standards. This ensures the highest purity and efficacy of Baloxavir Marboxil for your business needs. We understand the market demands rapid and effective solutions, and our commitment to excellence makes us the perfect partner. Choosing us means accessing a product that is not only scientifically backed but also competitively priced. Let’s work together to enhance your product lineup with Baloxavir Marboxil, and help your customers find relief from influenza faster than ever before!
Baloxavir Marboxil Manufacturer Custom Solutions,
In today's rapidly evolving pharmaceutical landscape, the demand for innovative antiviral treatments is stronger than ever. Among the promising solutions, Baloxavir Marboxil has emerged as a significant player, offering an effective option for the treatment of influenza. Manufacturers seeking to meet global procurement needs can benefit from customized solutions that align with market demands. Tailoring production processes, packaging, and supply chain logistics to fit specific client requirements can enhance the overall efficiency and effectiveness of distribution, ensuring that healthcare providers have timely access to critical medications. Flexibility and adaptability are essential in manufacturing, particularly when it comes to high-stakes pharmaceuticals like Baloxavir Marboxil. By embracing a collaborative approach with pharmaceutical partners, manufacturers can innovate their processes, optimizing formulations and delivery methods. This level of cooperation not only strengthens relationships but also contributes to faster market entry and improved patient outcomes across diverse geographical regions. Additionally, as regulatory landscapes continue to evolve, having a knowledgeable partner in the manufacturing process can be invaluable in navigating compliance and quality assurance challenges. Ultimately, the path to success in the global pharmaceutical market relies on the ability to customize solutions that meet the unique needs of buyers while maintaining the highest standards of quality and efficacy. With the ongoing rise in global health challenges, investing in tailored manufacturing solutions for Baloxavir Marboxil is not just a strategic advantage—it's a commitment to public health and patient care around the world.
Baloxavir Marboxil Manufacturer Custom Solutions
| Dimension | Description | Application | Regulatory Status | Formulation |
|---|---|---|---|---|
| Active Ingredient | Baloxavir Marboxil | Antiviral medication | FDA Approved | Tablet |
| Molecular Weight | 404.47 g/mol | Pharmaceutical research | Registered in EU | Suspension |
| Mechanism of Action | Inhibits viral replication | Influenza treatment | Clinical Trials Ongoing | Powder for Injection |
| Shelf Life | 24 months | Storage and transport | Post-Market Surveillance | Oral Tablet |
Related Products